This is part 3 of the blog series on the 5 outlooks of Environmental Forensics.
While giving the closing plenary at INEF2016 at the Örebro Castle in Örebro, Sweden, I add a point for the young (and older) researchers working in the field of environmental forensics.
Diagnostic Ratios
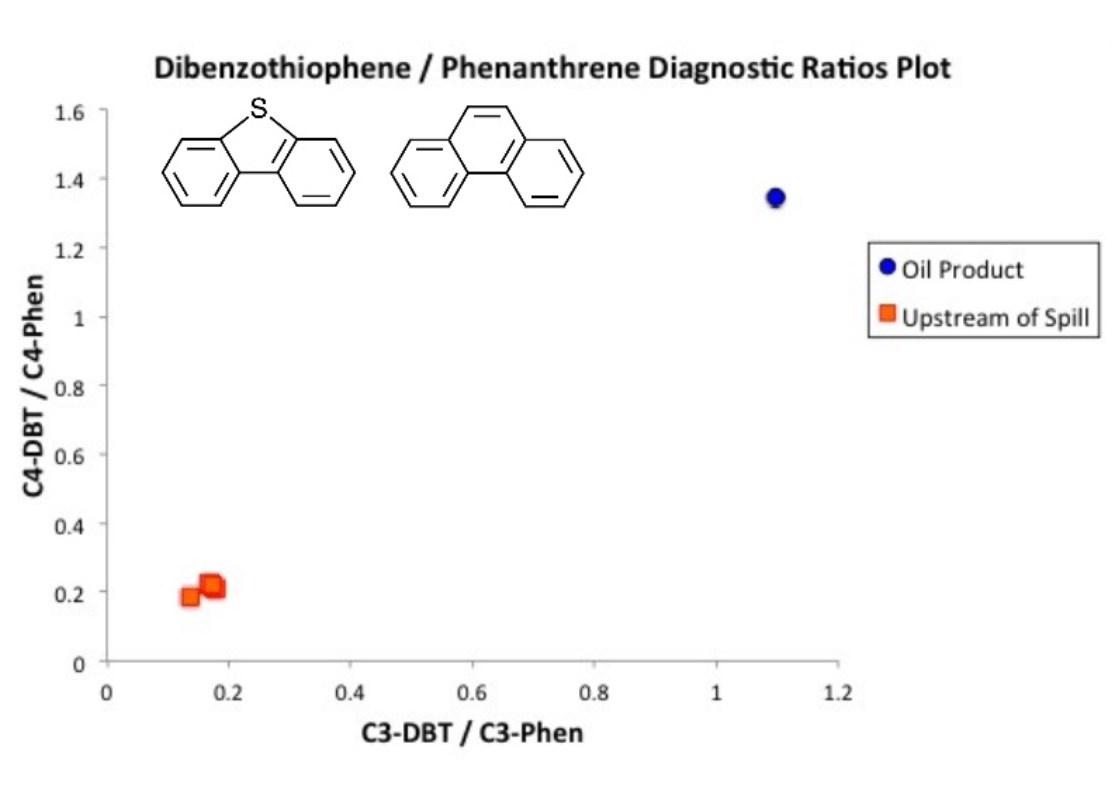
There have been many publications on the use of environmental forensics on a variety of different contaminants. One of the most common good of compounds analyzed in EFIs are the polycyclic aromatic hydrocarbons (PAHs). These compounds have many ‘diagnostic ratios’ that are used to determine the different sources that generated the PAHs.
6 Membered Ring vs 4 Membered Ring PAH Ratio
These ratios are founded in the literature and many have very solid and often overlooked science behind them. One plot that I have used extensively is the 6 membered ring PAH ratio (indeno-cd-pyrene over benzo-ghi-perylene) vs the 4 membered ring PAH ratio (fluoranthene over pyrene). This ratio works for a number of reasons that should be understood by the person generating the plots.
First, the ratio uses the same ‘sized’ PAHs to each other because their similar size and structure make them behave in the environmental similarly. If we released the two PAHs in equal amounts to the environment, they would ‘weather’ in a similar fashion. In other words, pyrene wouldn’t suddenly dissolve in water but fluoranthene would remain insoluble.
This means the ratio of these compounds that is emitted into the environment from whatever source it came from would stay relatively stable years later.
Choosing the Right Compounds
The two PAHs selected to ratio were picked for a very good reason. Looking closely at their structures, one of them has a 5 membered ring in the structure while the other has only 6 membered rings. Six membered rings allows that PAH to have complete charge resonance in its electron cloud while the 5 membered ring doesn’t allow complete resonance. This makes the 5 membered ring containing PAH less stable.
This can also be represented by the compounds heat of formation. Yunker et al (2002) examined this difference in heats of formation in their paper where these PAHs were selected for these ratios. By selecting two PAHs of similar size but picking the ones with the most different heats of formation, you get the PAHs that have the biggest difference in how they are formed which helps distinguish the process that went into making each of the PAHs. Hot, quick energy will form more of the kinetically favoured PAHs (ones with 5 rings) while slow, low temperature processes with preferentially form the thermodynamically stable PAHs (ones with aromatic 6 membered rings only).
Conclusion
The third take away from my plenary talk: 3. Know the fundamental principles behind the graphs and diagnostic ratios.
The next time you are plotting data based on some article you have read or figure you have seen, make sure to look up the fundamental chemistry that is driving that plot and what makes it work. Not only will it make more sense to you, but it may also allow you to communicate the results in a clear fashion, which touches upon my next take home message for my next blog: how to communicate scientific findings and analysis.
If you missed part 2 of this blog series, you can learn about environmental forensics investigations and regulatory compounds in my last blog post.